|
|
|
 |
DNA IS OUR LIFE |  |
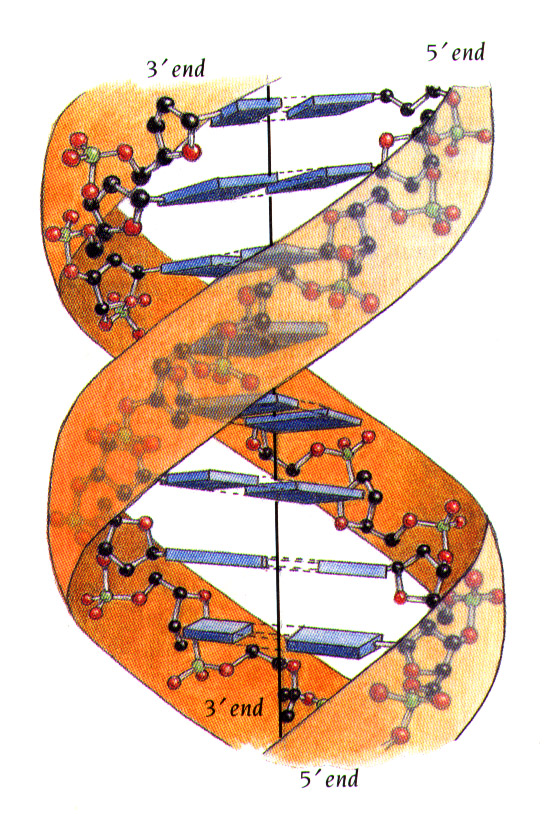
| DNA STRUCTURE The Structure of the DNA Molecule Access Excellence Classic Collection -------------------------------------------------------------------------------- Background Although scientists as far back in history as Aristotle recognized that the features of one generation are passed on to the next (...like begets like...) it was not until the 1860's that the fundamental principles of genetic inheritance were described by Gregor Mendel. Mendel's work with common garden peas, pisum sativum, led him to hypothesize that phenotypic traits (physical characteristics) are the result of the interaction of discrete particles, which we now call genes, and that both parents provide particles which make up the characteristics of the offspring. His theories were, however, widely disregarded by scientists of the time. In the last quarter of the 19th century, however, microscopists and cytologists, interested in the process of cell division, developed both the equipment and the methods needed to visualize chromosomes and their division in the processes of mitosis (A. Schneider, 1873) and of meiosis (E. Beneden, 1883). As the 20th century began many scientists noticed similarities in the theoretical behavior of Mendel's particles, and the visible behavior of the newly discovered chromosomes. It wasn't long before most scientists were convinced that the hereditary material responsible for giving living things their characteristic traits, and chromosomes must be one in the same. Yet, questions still remained. Chemical analysis of chromosomes showed them to be composed of both protein and DNA. Which substance carried the hereditary information? For many years most scientists favored the hypothesis that protein was the responsible molecule because of its comparative complexity when compared with DNA. After all, DNA is composed of a mere 4 subunits while protein is composed of 20, and DNA molecules are linear while proteins range from linear to multiply branched to globular. It appeared clear that the relatively simple structure of a DNA molecule could not carry all of the genetic information needed to account for the richly varied life in the world around us! It was not until the late 1940's and early 1950's that most biologists accepted the evidence showing that DNA must be the chromosomal component that carries hereditary information. One of the most convincing experiments was that of Alfred Hershey and Martha Chase who, in 1952, used radioactive labeling to reach this conclusion(See Graphics). This team of biologists grew a particular type of phage, known as T2, in the presence of two different radioactive labels so that the phage DNA incorporated radioactive phosphorus (32P), while the protein incorporated radioactive sulfur (35S). They then allowed the labeled phage particles to infect non-radioactive bacteria and asked a very simple question: which label would they find associated with the infected cell? Their analysis showed that most of the 32P-label was found inside of the cell, while most of the 35S was found outside. This suggested to them that the proteins of the T2 phage remained outside of the newly infected bacterium while the phage-derived DNA was injected into the cell. They then showed that the phage derived DNA caused the infected cells to produce new phage particles. This elegant work showed, conclusively, that DNA is the molecule which holds genetic information. Meanwhile, much of the scientific world was asking questions about the physical structure of the DNA molecule, and the relationship of that structure to its complex functioning. Watson and Crick In 1951, the then 23-year old biologist James Watson traveled from the United States to work with Francis Crick, an English physicist at the University of Cambridge. Crick was already using the process of X-ray crystallography to study the structure of protein molecules. Together, Watson and Crick used X-ray crystallography data, produced by Rosalind Franklin and Maurice Wilkins at King's College in London, to decipher DNA's structure. This is what they already knew from the work of many scientists, about the DNA molecule: DNA is made up of subunits which scientists called nucleotides. Each nucleotide is made up of a sugar, a phosphate and a base. There are 4 different bases in a DNA molecule: adenine (a purine) cytosine (a pyrimidine) guanine (a purine) thymine (a pyrimidine) The number of purine bases equals the number of pyrimidine bases The number of adenine bases equals the number of thymine bases The number of guanine bases equals the number of cytosine bases The basic structure of the DNA molecule is helical, with the bases being stacked on top of each other Working with nucleotide models made of wire, Watson and Crick attempted to put together the puzzle of DNA structure in such a way that their model would account for the variety of facts that they knew described the molecule. Once satisfied with their model, they published their hypothesis, entitled "Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid" in the British journal Nature (April 25, 1953. volume 171:737-738.) It is interesting to note that this paper has been cited over 800 times since its first appearance! Here are their words: "...This (DNA) structure has two helical chains each coiled round the same axis...Both chains follow right handed helices...the two chains run in opposite directions. ..The bases are on the inside of the helix and the phosphates on the outside..." "The novel feature of the structure is the manner in which the two chains are held together by the purine and pyrimidine bases... The (bases) are joined together in pairs, a single base from one chain being hydrogen-bonded to a single base from the other chain, so that the two lie side by side...One of the pair must be a purine and the other a pyrimidine for bonding to occur. ...Only specific pairs of bases can bond together. These pairs are: adenine (purine) with thymine (pyrimidine), and guanine (purine) with cytosine (pyrimidine)." "...in other words, if an adenine forms one member of a pair, on either chain, then on these assumptions the other member must be thymine; similarly for guanine and cytosine. The sequence of bases on a single chain does not appear to be restricted in any way. However, if only specific pairs of bases can be formed, it follows that if the sequence of bases on one chain is given, then the sequence on the other chain is automatically determined." and "...It has not escaped our notice that the specific pairing we have postulated immediately suggests a possible copying mechanism for the genetic material." And with these words, the way was made clear for tremendous strides in our understanding of the structure of DNA and, as a result our ability to work with and manipulate the information-rich DNA molecule. |
 |
|
| Discovery A New Drug Invitrogen buys into nanotechnology as R&D tool Invitrogen has announced two additional acquisitions in the area of nanotechnologcal tools for target and drug discovery, just as it completes the earlier-announced purchase of Biosource International. Fast method makes Abs for disease research UK scientists are pioneering a new technique to produce large numbers of antibodies quickly and reliably to help the study of dangerous bacteria. PET scans are early warning system for Alzheimer's PET scanning shows its worth in identifying patients with mild cognitive impairment that will go on to develop Alzheimer's disease. UK researchers move closer to Group B Strep vaccine Microbiologists believe they may be closer to developing a vaccine that can protect newborns from being infected by the potentially fatal bacteria Group B Streptococcus. Biotage completes distribution restructuring Swedish chromatography and microwave synthesis firm Biotage has taken some of its distribution into its own hands through the purchase of Swiss distributor Separtis Holding. Product News on Drug Discovery News by sponsor - Select a sponsor - Affymetrix Agilent Bio-Rad Bruker Daltonics CyBio Dionex Fujifilm Gilson Gyros HAMILTON Invitrogen Metrohm Niro A/S QIAGEN REMP Shimadzu Thermo Wyatt Analysis and instrumentation Assays and screening Compounds and consumables Contract services (outsourcing) Genomics / Proteomics Informatics & IT Lab equipment & consumables Liquid handling and automation Production technologies Separation and purification |
Gene Therapy can we live forever with gene therapy? |
|
More Pictures |
advantages of biotechnology UPCOMING FIELDS ANTISENSE TECHNOLOGY NEUROBIOLOGY RNA INFERENCE KNOCK OUT GENES CHROMOSOMAL WALKING DNA SEQUENCING venkatesh.perikala@gmail.com |
|